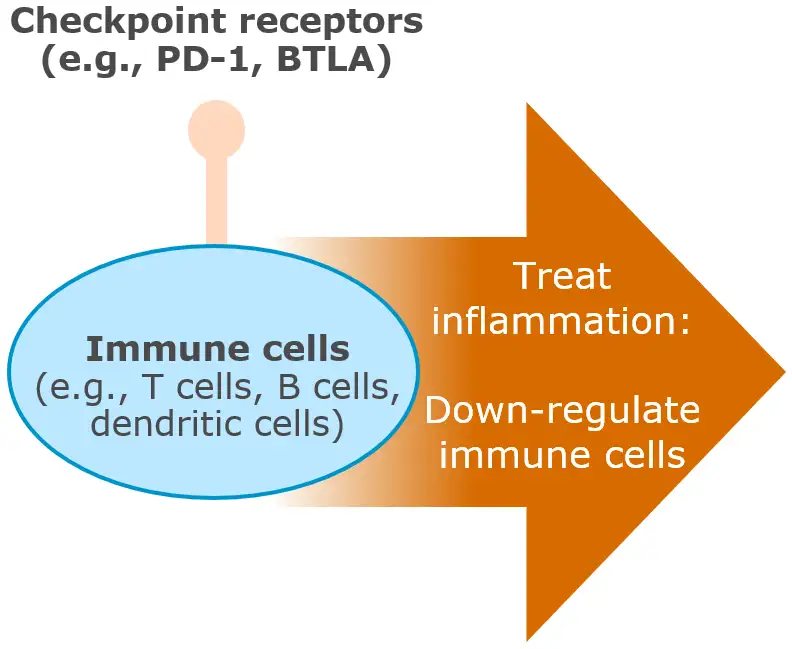
Immune cell modulators are a novel therapeutic class with the potential to deliver differentiated outcomes in autoimmune and inflammatory diseases including dermatology, gastroenterology, and rheumatology
-
Checkpoint agonists “hit the brakes” to restore immune balance and deliver differentiated outcomes
-
Membrane-proximal binding epitope and optimized Fc receptor binding affinity enables tight immune synapse and best-in-class potency
- Oral Presentation
Rosnilimab, a Novel PD-1 Agonist Monoclonal Antibody, Reduces T Cell Proliferation, Inflammatory Cytokine Secretion, and PD-1high Expressing CD4 and CD8 T Cells: Results From a Phase 1 Healthy Volunteer Clinical Trial
Luu et al., 2024
- Poster Presentation
Optimizing PD-1 Agonist Signaling with Membrane Proximal Binding of Rosnilimab, a Clinical Stage PD-1 Agonist IgG1 Antibody
Parmley et al., 2024
- Poster Presentation
Rosnilimab, a Novel PD-1 Agonist Monoclonal Antibody, Inhibits Peripheral T Cell Proliferation and Cytokine Secretion and Reduces Circulating PD-1 High Expressing CD4 and CD8 T Cells: Results from a Phase 1 Healthy Volunteer Clinical Trial
Luu et al., 2023
- Poster Presentation
Optimizing PD-1 Agonist Signaling with Membrane-Proximal Binding of Rosnilimab, a Clinical Stage PD-1 Agonist IgG1 Antibody
Parmley et al., 2023
- Poster Presentation
Rosnilimab, a Novel PD-1 Agonist Monoclonal Antibody, Demonstrated Modulation of Peripheral T Cell Activity and Reduction of Circulating PD-1 high Expressing CD4 and CD8 T Cells in a Phase 1 Healthy Volunteer Clinical Trial
Dahl et al., 2022
- Journal Publication
PD‑1‑Positive Cells Contribute to the Diagnosis of Inflammatory Bowel Disease and Can Aid in Predicting Response to Vedolizumab
Kim et al., 2023
- Journal Publication
Anti–PD-1 Antibodies Recognizing the Membrane Proximal Region are PD-1 Agonists that can Downregulate Inflammatory Diseases
Suzuki et al., 2023
- Journal Publication
PD-1 and PD-1 Ligands: From Discovery to Clinical Application
Okazaki et al., 2020
- Journal Publication
Immune Checkpoint Inhibitor PD-1 Pathway is Down-Regulated in Synovium at Various Stages of Rheumatoid Arthritis Disease Progression
Guo et al., 2018
- Journal Publication
Elevated Expression of PD‑1 on T Cells Correlates with Disease Activity in Rheumatoid Arthritis
Luu et al., 2018
- Journal Publication
Pathologically Expanded Peripheral T Helper Cell Subset Drives B Cells in Rheumatoid Arthritis
Rao et al., 2017
- Poster Presentation
ANB032, an Investigational B and T Cell Lymphocyte Attenuator (BTLA) Checkpoint Receptor Agonist, Modulates Dendritic Cell (DC) Maturation and Function: A Novel Mechanism Addressing Atopic Dermatitis Pathophysiology
Muench et al., 2024
- Poster Presentation
ANB032, a Novel BTLA Agonist Monoclonal Antibody, Inhibits T Cell Proliferation, Reduces Inflammatory Cytokines, and Down Modulates BTLA Expression on Circulating T and B Cells
Luu et al., 2024
- Poster Presentation
A Phase 2b, Randomized, Double Blind, Placebo Controlled, Global Study to Evaluate the Efficacy and Safety of ANB032 in the Treatment of Moderate to Severe Atopic Dermatitis
Ehst et al., 2023
- Poster Presentation
ANB032, a Novel BTLA Agonist Monoclonal Antibody, Inhibits T Cell Proliferation, Reduces Inflammatory Cytokines, and Down Modulates BTLA Expression on Circulating T and B Cells: Results from a First-in-Human Phase 1 Study
Luu et al., 2023
- Oral Presentation
ANB032, a Novel BTLA Agonist Monoclonal Antibody, Inhibits T Cell Proliferation, Reduces Inflammatory Cytokines, and Down Modulates BTLA Expression on Circulating T and B Cells: Results from a First-in-Human Phase 1 Study
Luu et al., 2023
- Poster Presentation
A Phase 2b, Randomized, Double Blind, Placebo Controlled, Multicenter, Global Study to Evaluate the Efficacy and Safety of ANB032 in the Treatment of Subjects with Moderate to Severe Atopic Dermatitis
Silverberg et al., 2023
- Poster Presentation
ANB032, a Novel BTLA Agonist Monoclonal Antibody, Inhibited T Cell Proliferation, Reduced Inflammatory Cytokines, and Down Modulated BTLA Expression on Circulating T and B Cells in Phase 1, Progresses Into a Phase 2 Study in Atopic Dermatitis
Luu et al., 2023
- Poster Presentation
ANB032, a Novel BTLA/HVEM Therapeutic Antibody, Inhibited T cells Derived from Atopic Dermatitis Patients In-vitro
Hsu et al., 2022
- Poster Presentation
Soluble BTLA (sBTLA) is Induced Following Targeting of BTLA In-vivo by ANB032, a Novel BTLA/HVEM Modulator Therapeutic Antibody for the Treatment of Autoimmune Disease
Dahl et al., 2021
- Poster Presentation
Discovery and Characterization of ANB032, a Novel BTLA/HVEM Checkpoint Modulator for Autoimmune/Inflammatory Disease
Dahl et al., 2020
- Journal Publication
BTLA Signaling in Conventional and Regulatory Lymphocytes Coordinately Tempers Humoral Immunity in the Intestinal Mucosa
Stienne et al., 2022
- Journal Publication
The Inhibitory Receptor B and T Lymphocyte Attenuator Controls γδ T Cell Homeostasis and Inflammatory Responses
Bekiaris et al., 2013
- Journal Publication
Therapeutic Potential of B and T Lymphocyte Attenuator Expressed on CD8+ T Cells for Contact Hypersensitivity
Nakagomi et al., 2013
- Journal Publication
Major Differences in Inflammatory Dendritic Cells and Their Products Distinguish Atopic Dermatitis From Psoriasis
- Poster Presentation
Discovery of a Novel High Affinity Anti-Human CD122 Antagonist Monoclonal Antibody (ANB033) that Abrogates IL-2 and IL-15 Signaling for the Treatment of T Cell-Mediated Inflammatory and Autoimmune Diseases
Hare et al., 2023
- Oral Presentation
Imsidolimab, an Anti-IL-36 Receptor Monoclonal Antibody, in the Treatment of Generalized Pustular Psoriasis: Results from a Phase 2 Trial
Gudjonsson et al., 2021
- Poster Presentation
A Phase 1 Study of ANB019, an Anti-Interleukin-36-Receptor (IL-36R) Monoclonal Antibody, in Healthy Volunteers
Khanskaya et al., 2018
- Journal Publication
Imsidolimab, an Anti-IL-36 Receptor Monoclonal Antibody for the Treatment of Generalised Pustular Psoriasis: Results from the Phase 2 GALLOP Trial
Warren et al., 2023
- Journal Publication
The Majority of Generalized Pustular Psoriasis Without Psoriasis Vulgaris Is Caused by Deficiency of Interleukin-36 Receptor Antagonist
Sugiura et al., 2013
- Journal Publication
IL-36 in Psoriasis
Towne et al., 2012
- Journal Publication
IL-36: a Potential Psoriasis Target?
Raison et al., 2012
- Journal Publication
Mutations in IL36RN/IL1F5 Are Associated with the Severe Episodic Inflammatory Skin Disease Known as Generalized Pustular Psoriasis
Onoufriadis et al., 2011
- Journal Publication
Interleukin-36–Receptor Antagonist Deficiency and Generalized Pustular Psoriasis
Marrakchi et al., 2011
- Journal Publication
IL-1F5, -F6, -F8, and -F9: A Novel IL-1 Family Signaling System That Is Active in Psoriasis and Promotes Keratinocyte Antimicrobial Peptide Expression
Johnston et al., 2011
- Journal Publication
Opposing Activities of Two Novel Members of the IL-1 Ligand Family Regulate Skin Inflammation
Blumberg et al., 2007
- Poster Presentation
Identification and Characterization of TSR-042, a Novel Anti-PD-1 Therapeutic Antibody
Laken et al., 2016
- Poster Presentation
Discovery of TSR-022, a Novel, Potent Anti-TIM-3 Therapeutic Antibody
Laken et al., 2016
- Poster Presentation
Targeting PD-1, TIM-3 and LAG-3 in Combination for Improved Immunotherapy Combinations
Kehry et al., 2015
- Poster Presentation
Generation of Anti-LAG-3 Monoclonal Antibodies for use in Immunotherapy Combinations
Jun et al., 2015
- Poster Presentation
Identification and Characterization of a Potent Anti-Human Tim-3 Antagonist
Correia et al., 2014
- Poster Presentation
Generation of Antagonistic Anti-TIM-3 and LAG-3 Monoclonal Antibodies for Potential Novel Immunotherapy Combinations
Jun et al., 2014
- Journal Publication
Preclinical Characterization of Dostarlimab, a Therapeutic Anti-PD-1 Antibody with Potent Activity to Enhance Immune Function in in Vitro Cellular Assays and in Vivo Animal Models
Kumar et al., 2021