


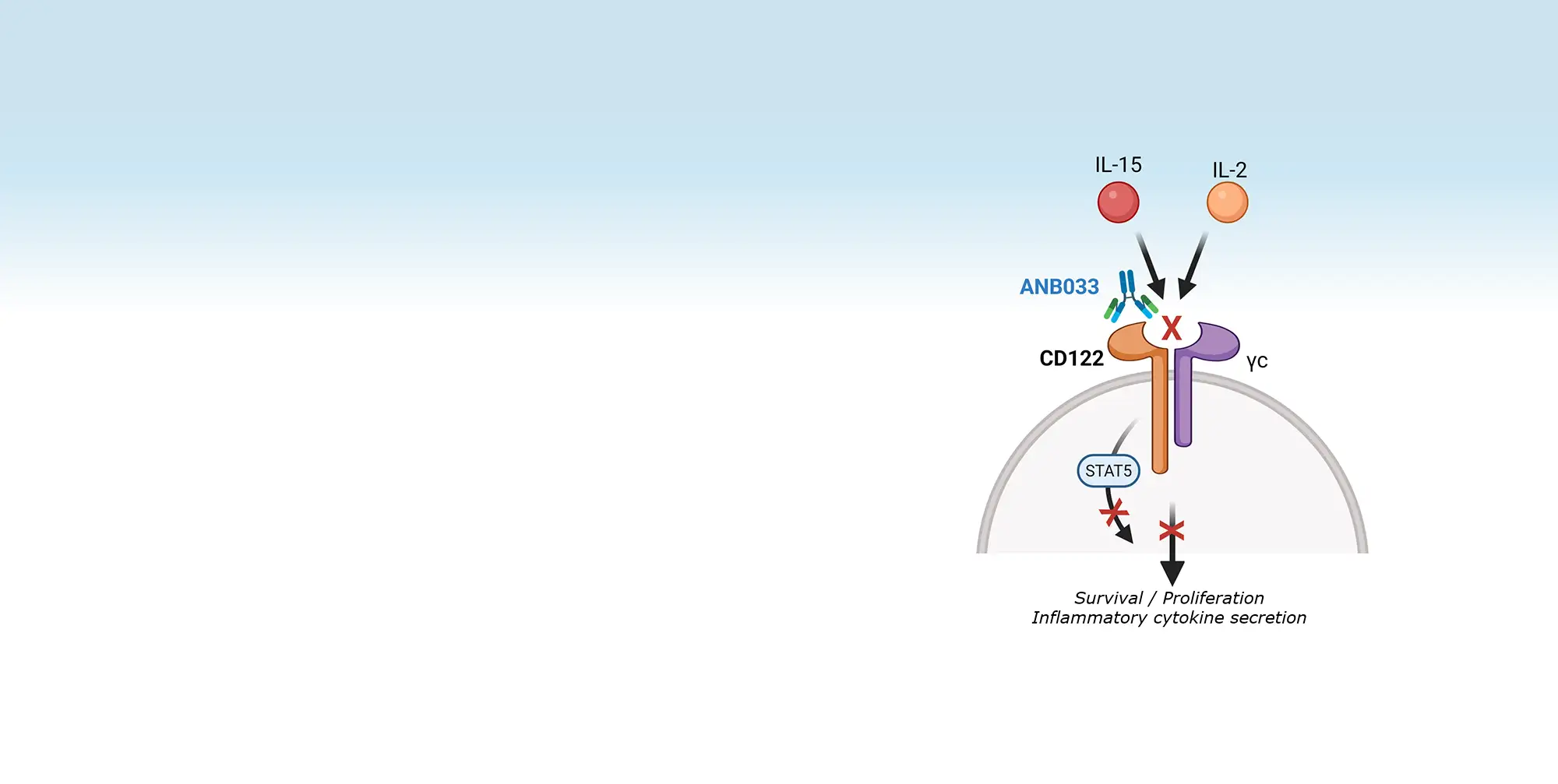
Press Releases
- Anaptys News
Anaptys Announces $100 Million Stock Repurchase Plan
AnaptysBio, Inc. (Nasdaq: ANAB), a clinical-stage biotechnology company focused on delivering innovative immunology therapeutics, today announced that its Board of Directors has authorized an amended Stock Repurchase Plan under which the Company may repurchase up to $100.0 million of the Company’s outstanding common stock, par value $0.001 per share. This amendment is in addition to the $6.4 million that remained as of Nov. 20, 2025 under the current $75.0 million Stock Repurchase Plan of which Anaptys has repurchased a total of 3,443,188 shares of common stock (11.2% shares outstanding before the start of this repurchase plan).
- Anaptys News
Anaptys Initiates Litigation Against Tesaro, a GSK Subsidiary
AnaptysBio, Inc. (Nasdaq: ANAB), a clinical-stage biotechnology company focused on delivering innovative immunology therapeutics, announced it has filed a Verified Complaint in Delaware Chancery Court, requesting a court declaration that TESARO, Inc. (“Tesaro”) has materially breached the parties’ Collaboration and Exclusive License Agreement (“Collaboration Agreement”) and that GSK (Tesaro’s corporate parent) has tortiously interfered with the Collaboration Agreement. Anaptys has requested that the court declare that Anaptys is entitled to all rights and remedies under the Collaboration Agreement.
- Anaptys News
Anaptys Announces Phase 2 Trial of Rosnilimab Did Not Meet Primary or Secondary Endpoints at Week 12 in Moderate-to-Severe Ulcerative Colitis
AnaptysBio, Inc. (Nasdaq: ANAB), a clinical-stage biotechnology company focused on delivering innovative immunology therapeutics, today announced that investigational rosnilimab was safe and well tolerated but did not meet the primary endpoint of mean change from baseline in modified Mayo Score (mMS) or key secondary endpoints of clinical response and clinical remission at Week 12 in the global Phase 2 trial for moderate-to-severe ulcerative colitis (UC). Placebo rates in the trial were within expected historical ranges. Given these results, this UC trial will be discontinued, resulting in at least $10 million in savings.