Rosnilimab — Rheumatoid Arthritis
Investigational rosnilimab has a potential best-in-disease profile in RA with a compelling safety and tolerability profile and JAK-like efficacy through six months that is durable for at least three months off-drug
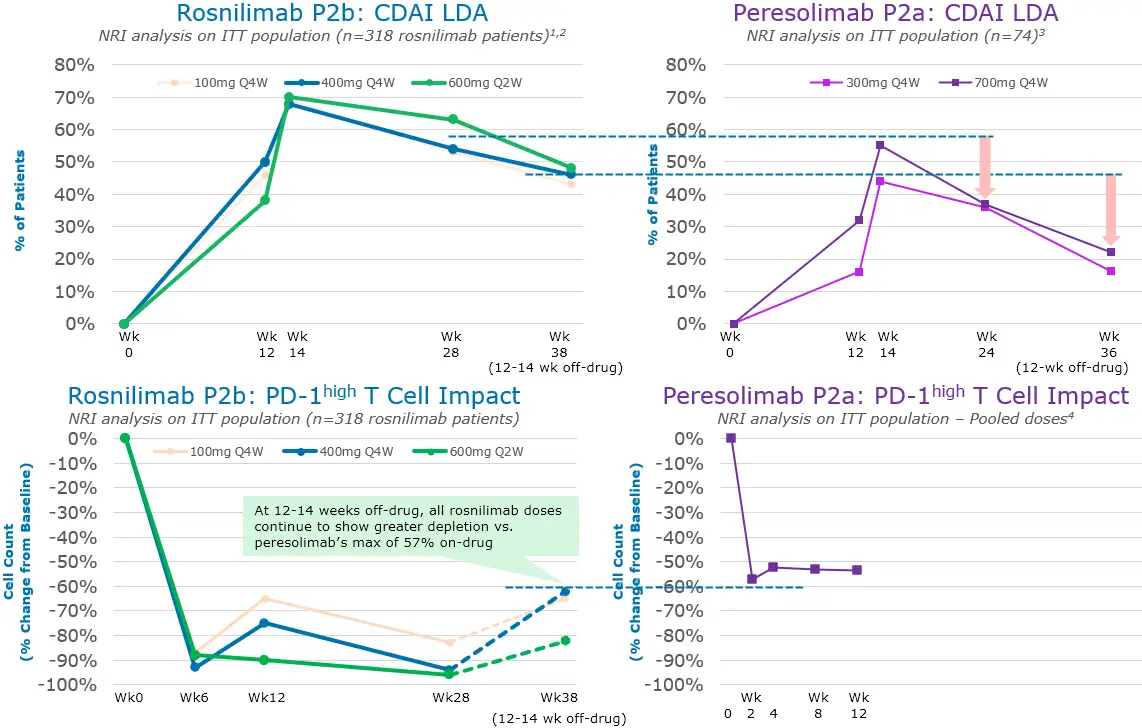
In a Phase 2b clinical trial of 424 patients, rosnilimab demonstrated rapid clinical and symptomatic improvement across all-comers, including difficult-to-treat patients, by three months that continued to improve through six months
The pathogenic T cell depleter was well tolerated with no treatment-related SAEs and no malignancies in more than ~185 patient years of rosnilimab exposure
Read about Phase 2b data here
Depleting pathogenic T cells broadly impacts multiple downstream, clinically validated drivers of RA pathogenesis
Adapted from Aletaha and Smolen, JAMA, 2018; 1. Chen et al, Clinical and Translational Immunology, 2024.
Rosnilimab demonstrates best-in-disease profile in RA

Rosnilimab, a pathogenic T cell depleter, is well-positioned for the ~$20 billion U.S. RA market which hasn’t had a new mechanism approved since 2012
For specific data, see press release and IR presentation.
1. Non-responder imputated (NRI) analysis on intent-to-treat (ITT) of all 318 rosnilimab patients randomized; 2. At Week 28, 53% (100mg Q4W), 54% (400mg Q4W), and 63% (600mg Q2W) rosnilimab patients were in CDAI LDA (57% pooled); 3. Tuttle et. al, NEJM, May 2023, Supplemental Appendix, At Week 28, 36% (300mg Q4W) and 37% (700mg Q4W) peresolimab patients were in CDAI LDA
To learn more about our clinical trial for Rheumatoid Arthritis please click here.