Rosnilimab — Rheumatoid Arthritis
In Q3 2023, we initiated a global Phase 2 trial of rosnilimab for the treatment of moderate-to-severe rheumatoid arthritis
Read about our June 2025 top-line data here
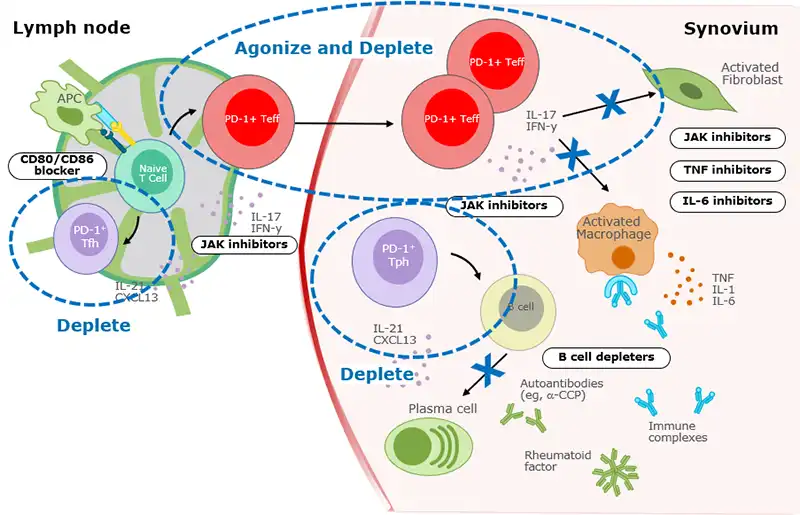
PD-1+ T cells broadly impact multiple clinically validated drivers of RA pathogenesis
>80% of T cells in RA synovium are PD-1+
- Similar findings are observed in treatment naïve and biologic experienced patients
Adapted from Aletaha and Smolen, JAMA, 2018
Rosnilimab Phase 2b data through six month in RA
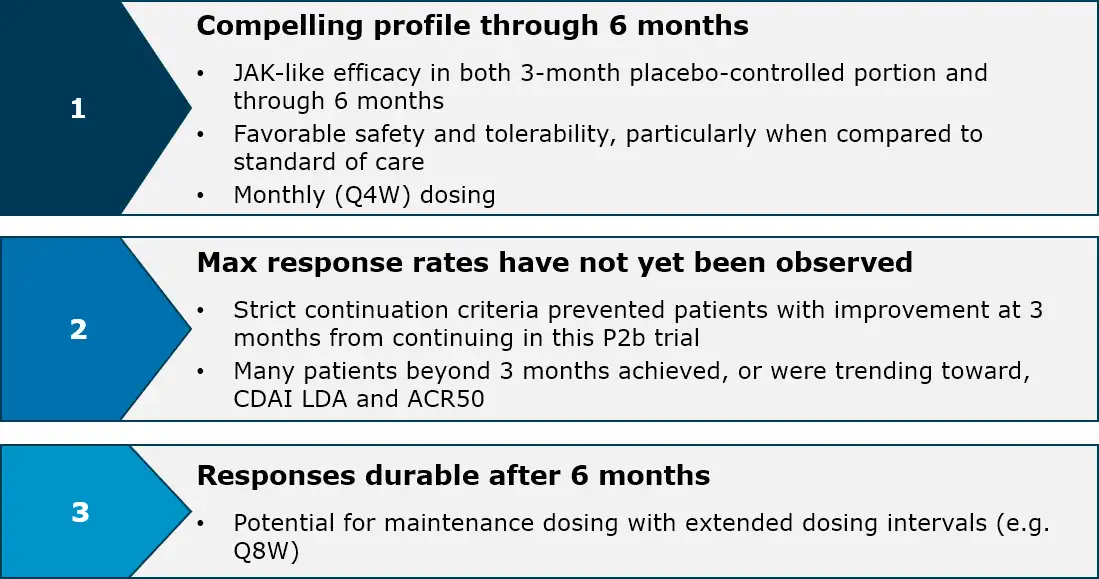
Rosnilimab, a best-in-class depleter and agonist targeting PD-1+ T cells, is well-positioned for the ~$20 billion U.S. RA market which hasn’t had a new mechanism approved since 2012
For specific data, see press release and IR presentation.
1. Non-responder imputated (NRI) analysis on intent-to-treat (ITT) of all 318 rosnilimab patients randomized; 2. At Week 28, 53% (100mg Q4W), 54% (400mg Q4W), and 63% (600mg Q2W) rosnilimab patients were in CDAI LDA (57% pooled); 3. Off-drug follow-up period ongoing; 4. Tuttle et. al, NEJM, May 2023, Supplemental Appendix, At Week 28, 36% (300mg Q4W) and 37% (700mg Q4W) peresolimab patients were in CDAI LDA
To learn more about our clinical trial for Rheumatoid Arthritis please click here.