Antibodies are complex proteins naturally generated by the immune system to neutralize foreign pathogens such as bacteria or viruses. B cells, a white blood cell type responsible for the generation of antibodies in response to pathogens, secrete billions of antibodies with different specificities into the bloodstream. Antibodies are structurally distinct Y-shaped proteins formed through the combination of two long proteins, called heavy chains, and two short proteins, called light chains. Each heavy and light chain pair forms a binding site where the antibody specifically binds its target, otherwise known as an antigen, at the Fab domain of the antibody molecule.
The specificity of each antibody to a target, and the potency of its binding strength to that target, are defined by the amino acid sequences of heavy and light chains in the Fab domain of the antibody molecule. The other end of the antibody, called the Fc domain, is responsible for communication between the antibody and the rest of the immune system. Fc domains bind to various receptors and cause immune system effector responses.
Therapeutic antibodies are typically non-naturally occurring, or recombinant, antibodies specifically developed to treat human diseases by binding to certain proteins, and thereby modulating key biological processes. Therapeutic antibodies are injectable products that are typically dosed subcutaneously or intravenously.
Our innovative platform is designed to replicate the natural process of somatic hypermutation (SHM) embedded within the human immune system to rapidly develop a diverse range of therapeutic-grade antibodies in vitro. SHM is a critical, endogenous process that generates the essential antibody diversity required to develop a natural immune response to pathogens. Our genomes encode a limited number of antibody genes, which are insufficient to generate antibodies against the wide variety of foreign pathogens encountered from the external environment. SHM enables our immune system to expand the limited diversity encoded within our genomes to the billions of antibody specificities required to defend ourselves against external pathogens.
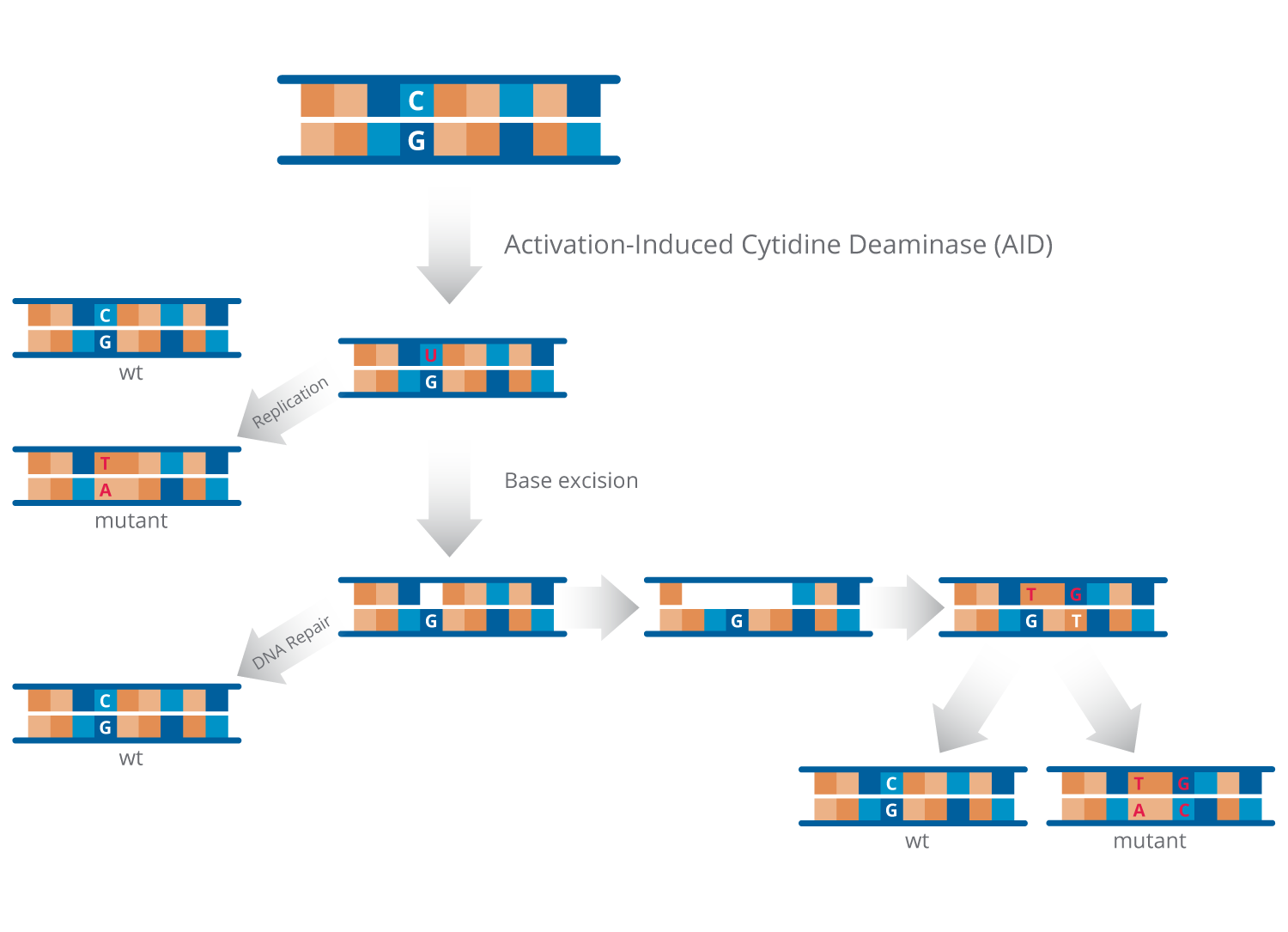
The key enzyme required for SHM is called activation-induced cytidine deaminase, or AID. AID has been genetically conserved throughout mammalian biology and is required for the non-random mutagenesis pattern associated with SHM. AID is specifically expressed by B cells after contact with a foreign pathogen and modifies antibody sequences in a non-random fashion. Through SHM, B cells evolve antibodies with the potency and specificity required to clear the foreign pathogen. However, within the in vivo environment, SHM does not generally progress to the creation of high potency antibodies or develop antibodies against the body’s own proteins.
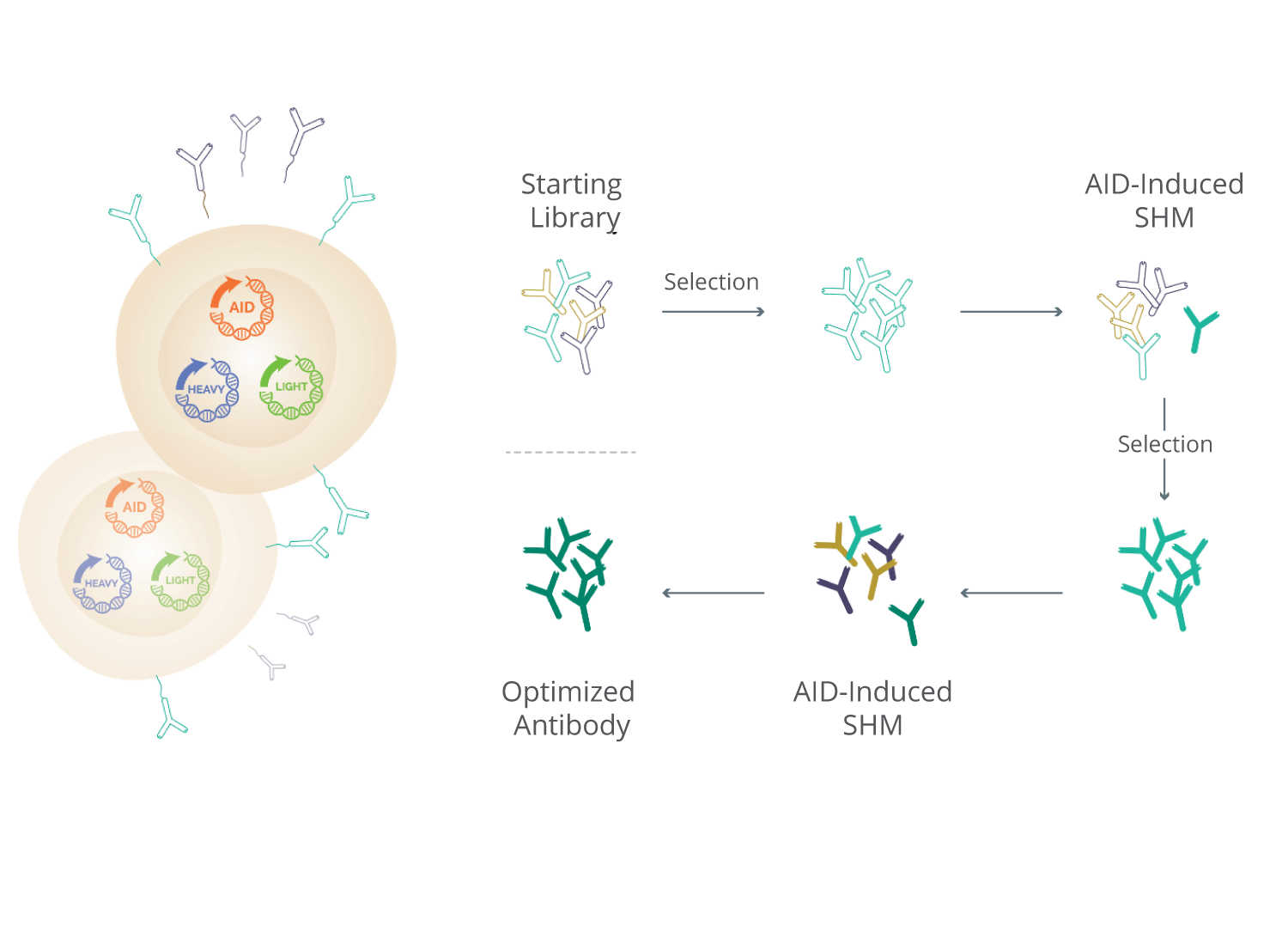
By coupling in vitro SHM with our mammalian cell system that simultaneously displays and secretes antibodies, we believe our SHM platform is able to rapidly identify and mature antibodies with desired functional activity to high potency while simultaneously mitigating the risks associated with manufacturing. We introduce AID into mammalian cells to replicate the non-random mutagenesis SHM pattern observed within B cells in vivo. Starting with a library of either fully-human or humanized antibodies, our platform generates AID-based variants of the starting antibody library throughout the process. We have demonstrated that the pattern of mutagenesis we observe in vitro using our platform technology closely mimics the pattern observed among in vivo generated antibodies, thereby increasing confidence that antibodies generated by our platform will be tolerated when used as therapeutic drugs in humans.
By selecting antibodies based on their antigen binding from the broad antibody library population our SHM platform develops, we are able to evolve in an iterative fashion the binding potency and function of antibodies to levels that we believe will be required for therapeutic use. We believe this approach allows us to rapidly generate antibodies with high binding potency against a target. Through this approach, we have successfully generated therapeutic antibody product candidates to more than 25 targets, including targets that have been challenging for competing antibody technology platforms.
Each evolving antibody is expressed within the SHM-active mammalian cell to concurrently (i) display the evolved antibody on the cell surface to permit cell sorting selection for potency properties while (ii) the same antibody is secreted into the extracellular media at sufficient quantities to permit functional assays to be conducted. In this manner, the evolving antibodies expressed by each transfected cell are assessed in a high-throughput fashion for the desired functional activity relevant to the therapeutic mechanism.
To access scientific publications on this topic, please click here.
Diversity Against Difficult Targets
We are able to generate an unprecedented diversity of antibodies by applying SHM-based diversification outside of the constraints of an in vivo environment. This enables us to develop antibodies against human targets that we believe have not otherwise been accessible to prior technologies.
High Potency
Because our platform generates highly-potent antibodies, we are potentially able to modulate every extracellular target associated with human disease, and believe only small therapeutic doses may be required to mediate therapeutic effect in vivo.
Functional Activity Selection
Our mammalian cell system simultaneously displays and secretes antibodies during the antibody discovery process, allowing us to incorporate functional assays throughout the process and focus on producing product candidates that are optimized for the desired therapeutic activity.
Speed
Our platform technology enables us to generate therapeutic-grade antibodies and initiate subsequent preclinical manufacturing and toxicology studies.
Manufacturability
By utilizing our mammalian cell display system, we believe our approach increases the probability of success in manufacturing and commercialization by mitigating risks associated with antibody expression, formulation and stability.
Pathologically Expanded Peripheral T Helper Cell Subset Drives B Cells in Rheumatoid Arthritis
Rao et al., 2017
Elevated Expression of PD‑1 on T Cells Correlates with Disease Activity in Rheumatoid Arthritis
Luu et al., 2018
Immune Checkpoint Inhibitor PD-1 Pathway is Down-Regulated in Synovium at Various Stages of Rheumatoid Arthritis Disease Progression
Guo et al., 2018
PD-1 and PD-1 Ligands: From Discovery to Clinical Application
Okazaki et al., 2020
Anti–PD-1 Antibodies Recognizing the Membrane Proximal Region are PD-1 Agonists that can Downregulate Inflammatory Diseases
Suzuki et al., 2023
PD‑1‑Positive Cells Contribute to the Diagnosis of Inflammatory Bowel Disease and Can Aid in Predicting Response to Vedolizumab
Kim et al., 2023
Optimizing PD-1 Agonist Signaling with Membrane-Proximal Binding of Rosnilimab, a Clinical Stage PD-1 Agonist IgG1 Antibody
Parmley et al., 2023
Optimizing PD-1 Agonist Signaling with Membrane Proximal Binding of Rosnilimab, a Clinical Stage PD-1 Agonist IgG1 Antibody
Parmley et al., 2024
Therapeutic Potential of B and T Lymphocyte Attenuator Expressed on CD8+ T Cells for Contact Hypersensitivity
Nakagomi et al., 2013
The Inhibitory Receptor B and T Lymphocyte Attenuator Controls γδ T Cell Homeostasis and Inflammatory Responses
Bekiaris et al., 2013
BTLA Signaling in Conventional and Regulatory Lymphocytes Coordinately Tempers Humoral Immunity in the Intestinal Mucosa
Stienne et al., 2022
Discovery and Characterization of ANB032, a Novel BTLA/HVEM Checkpoint Modulator for Autoimmune/Inflammatory Disease
Dahl et al., 2020
ANB032, a Novel BTLA/HVEM Therapeutic Antibody, Inhibited T cells Derived from Atopic Dermatitis Patients In-vitro
Hsu et al., 2021
Opposing Activities of Two Novel Members of the IL-1 Ligand Family Regulate Skin Inflammation
Blumberg et al., 2007
IL-1F5, -F6, -F8, and -F9: A Novel IL-1 Family Signaling System That Is Active in Psoriasis and Promotes Keratinocyte Antimicrobial Peptide Expression
Johnston et al., 2011
Interleukin-36–Receptor Antagonist Deficiency and Generalized Pustular Psoriasis
Marrakchi et al., 2011
Mutations in IL36RN/IL1F5 Are Associated with the Severe Episodic Inflammatory Skin Disease Known as Generalized Pustular Psoriasis
Onoufriadis et al., 2011
IL-36: a Potential Psoriasis Target?
Raison et al., 2012
IL-36 in Psoriasis
Towne et al., 2012
The Majority of Generalized Pustular Psoriasis Without Psoriasis Vulgaris Is Caused by Deficiency of Interleukin-36 Receptor Antagonist
Sugiura et al., 2013
Imsidolimab, an Anti-IL-36 Receptor Monoclonal Antibody for the Treatment of Generalised Pustular Psoriasis: Results from the Phase 2 GALLOP Trial
Warren et al., 2023
A Phase 1 Study of ANB019, an Anti-Interleukin-36-Receptor (IL-36R) Monoclonal Antibody, in Healthy Volunteers
Khanskaya et al., 2018
Generation and Iterative Affinity Maturation of Antibodies in Vitro Using Hypermutating B-Cell Lines
Cumbers et al., 2002
Intricate Targeting of Immunoglobulin Somatic Hypermutation Maximizes the Efficiency of Affinity Maturation
Zheng et al., 2005
The Biochemistry of Somatic Hypermutation
Peled et al., 2005
AID-Induced Insertions and Deletions Complement Point Mutations to Massively Expand the Diversity Created by Somatic Hypermutation of Antibodies
Verdino et al., 2015
High Affinity Humanized Antibodies without Making Hybridomas; Immunization Paired with Mammalian Cell Display and In Vitro Somatic Hypermutation
McConnell et al., 2012
Humanization of Antibodies Using Heavy Chain Complementarity-Determining Region 3 Grafting Coupled with in Vitro Somatic Hypermutation
Bowers et al., 2013
Generation of Antagonistic Anti-TIM-3 and LAG-3 Monoclonal Antibodies for Potential Novel Immunotherapy Combinations
Jun et al., 2014
Identification and Characterization of a Potent Anti-Human Tim-3 Antagonist
Correia et al., 2014
Generation of Anti-LAG-3 Monoclonal Antibodies for use in Immunotherapy Combinations
Jun et al., 2015
Targeting PD-1, TIM-3 and LAG-3 in Combination for Improved Immunotherapy Combinations
Kehry et al., 2015
Discovery of TSR-022, a Novel, Potent Anti-TIM-3 Therapeutic Antibody
Laken et al., 2016