ANB032 — Overview
ANB032 is a novel, non-depleting antibody that binds to the BTLA checkpoint receptor and inhibits activated T cell proliferation, reduces inflammatory cytokine secretion and modulates dendritic cell (DC) function, including inducing Tregs, both in inflamed tissue and the periphery
ANB032 has potential to treat a wide range of systemic inflammatory diseases
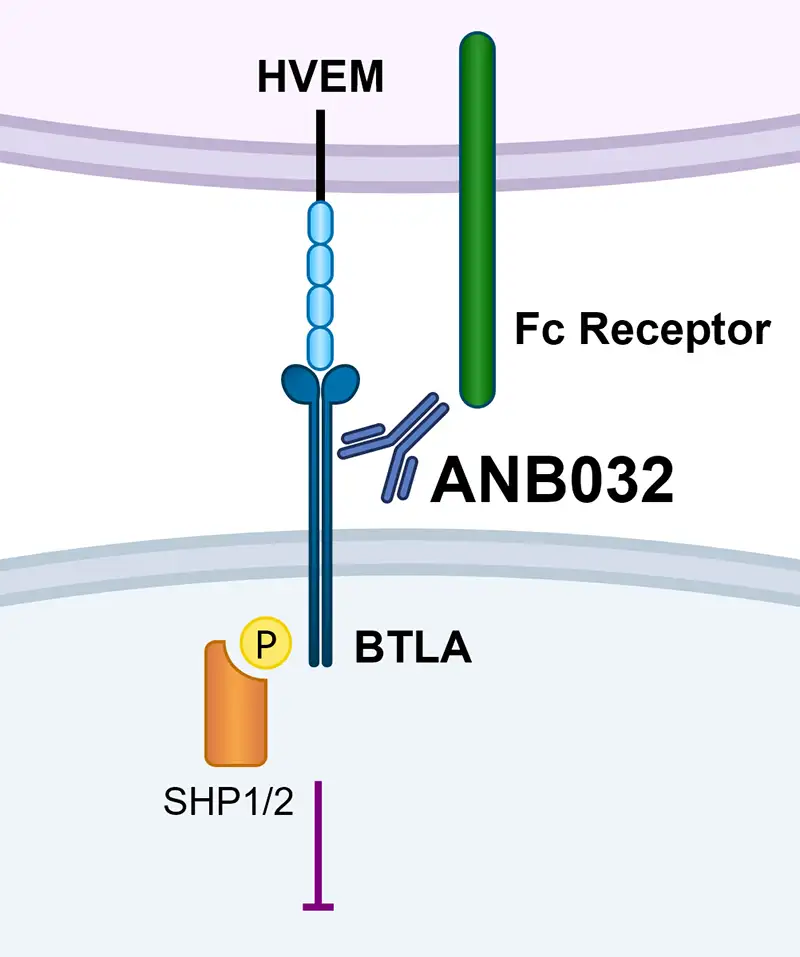
ANB032: IgG4 antibody (non-depleting)
- Binds to BTLA on epitope proximal to immune cell
- Fc receptor binding profile contributes to differentiated potency
- Non-blocking of HVEM engagement with optimized antigen binding affinity
ANB032’s agonist signal modulates immune cells
- Inhibits activated T cell proliferation
- Reduces inflammatory cytokine secretion
- Modulates DC function, including inducing Tregs
BTLA is key node of immune regulation
- B and T lymphocyte attenuator (BTLA) is a potent modulator of T cells, B cells and dendritic cells (DC)
- Expressed only on immune cells and preferentially on activated immune cells
- Dysregulation of BTLA pathway accelerates onset and exacerbates disease
Jurkat BTLA SHP2 Recruitment Assay methodology: BTLA and SHP2 are fused with complementary enzyme fragments, when SHP2 is recruited to activated phosphorylated BTLA, the enzyme donor and enzyme acceptor form active β-gal that is detected by chemiluminescence.
ANB032 demonstrated favorable safety and tolerability with rapid and sustained PK/PD activity
96 healthy volunteers in SAD and MAD cohorts in Phase 1 study
- Favorable ~2-week half-life with IV and SQ dosing
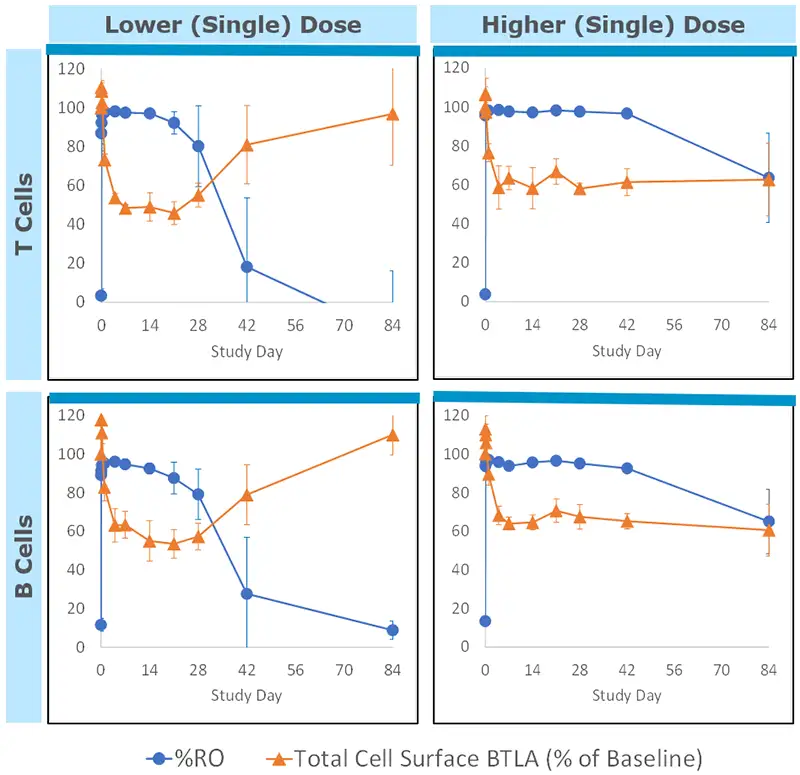
- Full receptor occupancy (RO) within hours and maintained for >30 days
Rapid and sustained target engagement on both T and B cells
- Duration of reduced BTLA expression persisted in dose-dependent manner
Well-tolerated with no dose limiting tox
- No SAEs
- Most AEs mild-to-moderate, short duration, dose independent and resolved without sequelae
- No evidence of infection risk or cancer risk to date
To access scientific publications on this topic, please click here.
To learn more about our clinical trial for Atopic Dermatitis please click here.